Dilution Calculator Percent – Acid / Alcohol Liquid Gallons
Are you searching for the best Dilution Calculator Percent? If yes, then here we guide you with authentic information and also provide you with other details which will be helpful for you. A dilution refers to making a unique lower concentration solution from a higher level concentration.
Keep in mind that solutions will usually be stored only at a higher level of concentration. This is happened because for the convenient for use and also avoids contamination.
Moreover, this calculator can be used to perform specific dilution calculations when you are working with percent % or molar solutions. The most important thing for you is to remember that when you are working with percent solutions, the overall concentration unit must be the percent for both stocks. Both stocks mean final diluted solution and concentrated solution.
How is Dilution Calculator Percent work?
Every cell of the calculator corresponds to a term in the formula which is presented below. All you need to do is enter the appropriate values in every cell except that one which you want to calculate.
Keep in mind that you need to fill all the given cells and you can leave any cell blank. The blank cell value will be only calculated based on the other cell’s value. When the calculation is complete, the calculated cell value will be highlighted.
Moreover, keep in mind that subsequent calculations will only calculate the highlighted cell value. Therefore, the blank cell has a complete priority over the other highlighted cell. For your convenience, the dilution calculator percent will especially allow you to select the concentration units and another different volume.
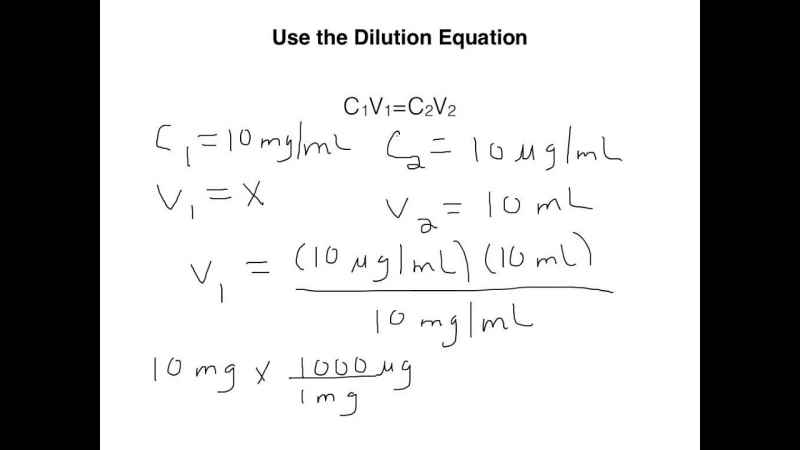
What is the Dilution Equation?
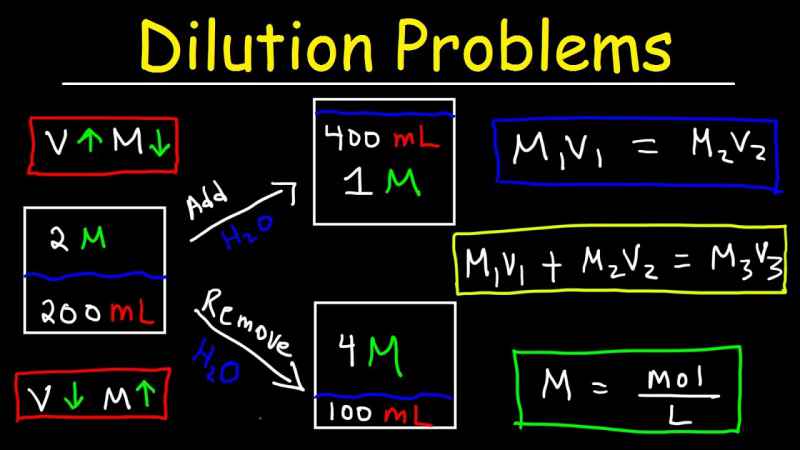
C1V1 = C2V2
- Where C1 is the total concentration of the entire stock solution.
- Where V1 is the specific volume to be removed from only the concentrated solution of stock.
- Where C2 is the final total concentration of the diluted solution.
- Where V2 is the only complete volume of the solution. Also, keep in mind that this is the specific volume that results get after V1 from the overall stock solution. Moreover, it has been diluted with the diluent to specific achieve the total volume of diluted V2.
Note:
A commonly used notation and best alternative for this dilution equation are M1V1 = M2V2, where M is used only in place of C.
Frequently Asked Questions of Dilution Calculator Percent
1. The total HCL solution stock concentration is 5mol/L, 2ml is used to produce a total dilute solution of 500ml, so what is the concentration of the diluted solution?
Answer. Concentration (dilute) = 5mol/L * 2ml / (500ml)
Which is equal to 0.02mol/L.
2. The total diluted solution is only 300ml, with a 100mM concentration. Then how much 5M stock of HCL solution is needed?
Answer. Volume(Stock) = 300ml * 100mM / 5M
Which is equal to 6ml.
Conclusion
Now you don’t need to worry about that how Dilution Calculator Percent works, because now you get all the authentic details and information which can enhance your knowledge.
But keep in mind that you need to leave one blank cell and this cell will calculate your desired dilute solution based on your other input values. So, now you can easily calculate the dilute percent with the given equation.



I am happy to find a website that shows its full development.